Foxtrot protocol
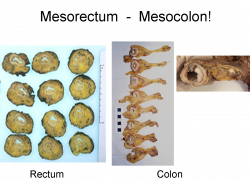
The overall survival of colonic cancer is now less than that of rectal cancer both in the Yorkshire cancer registry data and in Swedish data. Following observations in the MRC Clasicc study and courses on colonic surgery that PQ has taught on at the Karolinska Hospital, Sweden it is apparent that, as is the case in rectal cancer, there is marked variation in the quality of surgery of colorectal cancers. This variation takes the form of incomplete removal of the mesocolon and its lymphatic supply, different lengths of resection to the high tie lymph node and different clearances of the surgically created mesocolic resection margin e.g. caecum and ascending colon (Bateman et al).
The FOxTROT study provides a unique opportunity to prospectively evaluate resectional quality and its influence on outcome for colon cancer. The trial management committee contains the necessary surgical, histological and radiological expertise to complete this important aspect of the study. Future trials of adjuvant and neoadjuvant chemotherapy will gain considerable benefit, if this study can establish criteria for resectional quality.
This trial is capable of evaluating the effect of neo-adjuvant chemotherapy upon the tumour as assessed by histology, but can also assess the absolute quality of colonic surgery and the potential influence of neoadjuvant therapy upon it.
This study will use TNM 5 not TNM 6 as the latter is not recommended by the Royal College of Pathologists. The added advantage is that MRC Clasicc and MRC CR07 used TNM5 criteria and inter-trial analysis will therefore be possible.A secondary analysis of Clasicc colon resections is being completed and will be used to further inform the histology analysis in the proposed study
We will receive photographs of the specimens to independently judge the quality of surgery and duplicate sections of the histology. We will collect standard data on pathology, complete resection and importantly the effects of chemotherapy. We also propose that the histology is centrally reviewed and digitised to create a unique archive to quality control the study and to allow further research in this unique trial.
Classification
The MRC Clasicc and CR07 histological criteria have been demonstrated to be usable in the context of phase III clinical trials and an early publication demonstrated their value in rectal cancer (Nagetaal et al 2002).
Grading
We recommend four grades of mesocolon resection quality:
Mesocolic plane with hie tie: Mesocolic tissue removed intact with no lacerations of the mesocolic surface and with a distinct high tir pedicle present
Mesocolic plane: Mesocolic tissue removed intact with no lacerations of the mesocolic surface.
Intramesocolic plane: Mesocolic tissue largely intact but the mesocolon shows irregularity/laceration/defects that do not reach down to the muscularis propria.
Muscularis propria plane : Mesocolic tissue extensively disrupted with marked irregularity of the mesocolon. The surgical margin may extend down onto the muscularis propria in mesocolic areas. Please note that this feature should only be assessed on the areas covered in mesocolon and not in the areas where peritoneum is closely adherent to the muscularis propria.
Mesocolic resection margins will be assessed with a margin of less than 1 mm determining involvement. Distance of tumour to the mesocolic resection margin will also be given.
Regression grading and complete response
Two methods will be trialled. Dr Warren with Dr Wheeler (Wheeler et al 2002) have published a 3 grade simplified regression scheme and we would use this in association with the Dworak modification of Mandard grading. This comparison would be valuable and retaining Dworak/Mandard will allow us to compare this study to those of rectal cancer. Assessment of complete response will be by the Core method (Core protocol) where 5 blocks of tumours are taken initially. If no tumour is found, the entire area is blocked and if tumour is still absent these blocks are levelled. If no tumour is seen at this point then the tumour has undergone a complete response.
